In 2023, a Danish group published a statistical analysis showing disproportionate adverse events for early Pfizer/BioNTech vaccines. The graph of blue, green and yellow dots was widely circulated.
Figure 1: Graph of Danish batches by adverse events reported compared to doses shipped, grouped and colour coded
The behind the scenes story is one of lack of transparency, regulatory oversight, and manufacturing consistency. The critiques of the paper are easily refuted. Interestingly, the most prominent critique came from a 23 year old computer science student. Perhaps he’s a polymath genius or perhaps the evidence points to him being a ghostwriter being used by an industry renowned for such behaviour.
How the story unfolded
One of the authors, Vibeke Maniche, published her work on Twitter at 7:13am on 11th April 2023. Within 12 minutes, Anders Hviid, Professor of epidemiology at the Serum Institute and a key figure regarding vaccine safety surveillance in Denmark, wrote, “FRAUD or incompetence?...This is a case of deliberate misinformation.“ When independent scientists have taken the time to collate a huge dataset by batch number and carried out detailed analysis, such a response from a fellow professional seems rash to say the least.
He then wrote to the editors at the journal pressuring them to retract. The editors stood their ground and forwarded the critique to the authors who were able to defend each point successfully. He was invited to write a letter which was published on 15th July and made the point that shipped doses might not all have been given. Before his letter was published a separate letter was submitted authored not by him but by a single author, Borja Somovilla Del Saz, the 23 year old computer science student.
What was going on?
Who is Borja Somovilla del Saz?
In his July 2023 paper, Borja Somovilla del Saz argued that the higher adverse event rates in early batches were due to their distribution to high-risk populations, including healthcare workers and the elderly. When de Welt asked BioNTech for comment they referenced his paper saying, “there is nothing more to say than this.” How odd that a pharmaceutical company has nothing more to add than what was penned by a 23 year old computer student? The Paul-Ehrlich-Institute in Germany also referenced his paper. Stranger still. Is he a polymath genius or is something else going on?
On Reserach Gate (where academics share their publications) he lists his skills as “computer networking”. Elsewhere he advertises for tutoring in computer programming. He earned his degree from Universidad Del Pais Vasco but claims affiliation to Valencia University where he lives and has an academic email address. He has a Twitter account which links to a Spanish blog.
His papers on Research Gate include the following:
A follow up critique of the Florida Surgeon General’s findings:
"Reconsidering the inclusion of Ladapo’s work in the meta-analysis: Validity concerns and implications" Feb 2024 (Accepted in 2 months)
A critique of the Florida Department of Health publication accusing them of bias when reporting on cardiac deaths in males:
"Questionable robustness in the findings of a meta-analysis" Aug 2023 (Accepted in 17 days)
His letter regarding the Danish batch paper:
“Batch-dependent safety of the BNT162b2 mRNA COVID-19 vaccine.”
Spanish commentary on covid vaccines:
"Evidence supporting the use of covid vaccine (Update: 25/12/2022)"
Critique of the Malhotra paper
"Comment on Malhotra et al (2022): Curing the pandemic misinformation with more misinformation?" (Preprint only)
Paper critiquing the assertion that abortion has a clear negative impact on mental health.
“Critique of Coleman's new study: "The Turnaway Study: A case of self-correction in science upended by political motivation and unverified findings."”
The last one is interesting. This paper adopts the style of a social scientist, characterized by verbose and flowery language, extensive use of lengthy quotations, use of academic vocabulary at the expense of clarity and a focus on critiquing theoretical frameworks and study design rather than engaging in rigorous data analysis or presenting novel empirical findings.
His earlier publications—written in Spanish—focused on unrelated topics such as LGBTQ+ rights and other abortion issues. Despite the global vaccine debate throughout 2021 and early 2022, Somovilla del Saz published nothing on the topic until late 2022. His first covid paper critique followed Aseem Malhotra’s high-profile publication - one of the first peer-reviewed papers critical of mRNA vaccines, suggesting a reactionary motive. His earlier papers were all written in Spanish until September 2022 when he switched to English. There are other key differences between his covid papers and previous papers.
His Work and Writing Style
His writing style reveals inconsistencies:
Earlier works, such as his Response to Catholic.net (2021), include conversational phrases like "Let’s see the claims" and "this doesn’t hold up," alongside frequent omissions of articles, such as "review of studies shows lack of..." These informal features contrast sharply with later works, such as his critique of Malhotra (2022), which employs polished academic language, including verbs like "underscores" and "demonstrates," alongside formal use of articles like "the" and "a."
Similarly, while the Malhotra Critique (2022) uses the Oxford comma rigorously, earlier works like the Response to Catholic.net omit it entirely. In later works there is hyphenation of phrases like “non-peer-reviewed”, "self-controlled" whereas in the Turnaway Critique (2022), hyphenation is almost absent.
Even within individual articles, inconsistencies emerge. For example, in the Ladapo critique (2023), hyphenation varies between "non-peer-reviewed" and "non peer reviewed", and the tone alternates between formal and conversational as if there was more than one author.
Such inconsistencies, combined with the timing of his critiques, suggest possible involvement of external parties. Ghostwriting or the strategic use of external voices is not uncommon in the pharmaceutical industry. Companies often use third parties to deliver messages that align with their interests, especially in contentious areas like vaccine safety. Was this the case here?
Scientific debate?
It is quite right for scientists to be held to account for their work but what was happening here was different. The key finding was that the mortality rate for "Blue Batches" accounted for just 4% of doses but were linked to 50% of vaccine-related deaths. These high-risk "blue" batches were withdrawn after only 80,000 doses—far smaller than the standard batch size of one million doses. The smaller distribution of the flagged batches has been confirmed. Was it an intentional precaution, a production anomaly, or pulling the batches once problems were evidence? If these batches were indeed limited in size, regulators have yet to provide a clear justification for this deviation from normal distribution practices. Although the earlier batches were smaller, it seems highly unlikely they would have been over twelve times smaller.
The main retort was that this was due to the frail being injected with earlier doses and a greater number of coincidental deaths being reported and that early batches had a smaller batch size for logistical reasons. Neither of these claims stands up to scrutiny. The proportion aged over 70 years was only 21% for these early batches, a ratio that was similar to previous batches. Besides, when elderly frail people die the reporting rates are far lower than for younger people where death is unexpected. Another claim was that healthcare professionals were more likely to report their own conditions than those in their patients. Given that there was a 1,104 fold increased reporting of deaths between the worst and least harmful batch, it is quite the claim that healthcare workers were reporting on their own deaths more than their patients’.
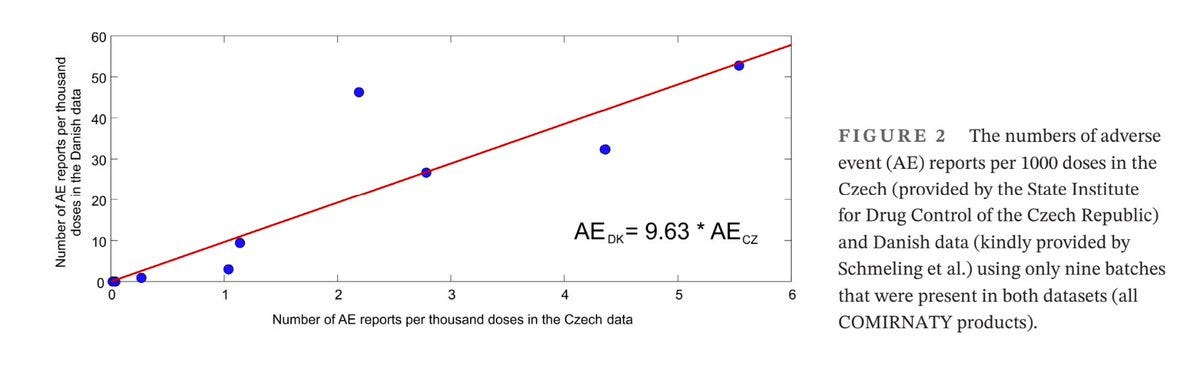
Adding to the picture, a Czech Republic study independently confirmed the Danish findings, identifying significant overlaps in problematic batches. Swedish data is published and confirms this pattern, validating the results across multiple datasets. Notably, Pfizer’s own internal safety report flagged the same nine batches among the 20 most problematic for adverse events. These findings indicate a systemic issue with certain early batches that cannot be easily explained by demographic factors or heightened awareness.
Figure 2: Adverse events reporting rate for each of the nine batches used in both Denmark and the Czech Republic
Manufacturing and Production Sites
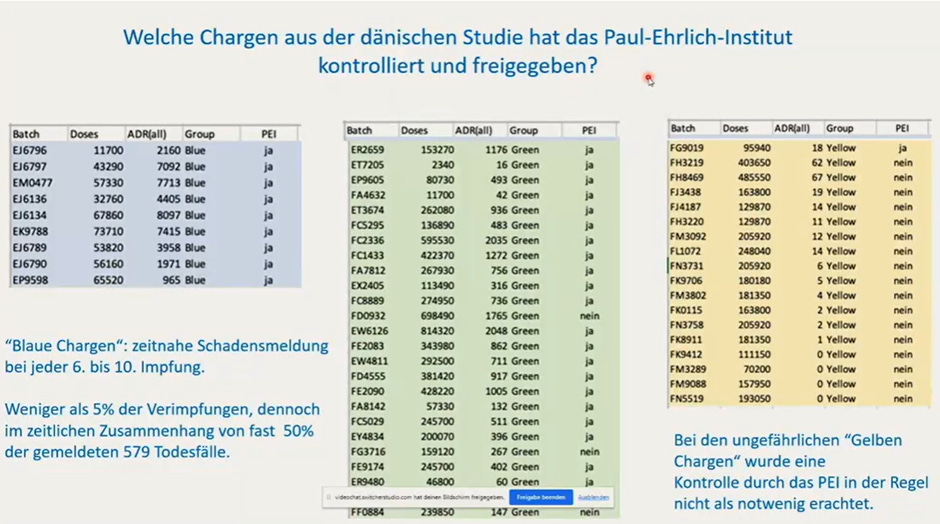
The batch discrepancies also point to potential differences in manufacturing processes. Initially, Pfizer produced mRNA at its Andover, Massachusetts facility before production shifted to BioNTech’s Marburg, Germany site in mid-2021. While the Marburg facility flagged several problematic batches for further testing, many of the "yellow" batches associated with the fewest adverse events were not subjected to the same quality control measures. How did they know which to test?
Figure 3: German table showing whether PEI had carried out testing in final column - colour coded by colour allocation in Danish paper
Abortions for Fetal Anomalies
Compounding concerns, data from England and Wales revealed a spike in abortions for fetal anomalies between March and May 2021, a period when these procedures were significantly above expected levels. Women vaccinated in their first trimester with the early "blue" batches would have reached the 20-week anomaly scan period during this timeframe. While no causal link has been definitively established, the timing of these findings warrants further investigation into potential connections between batch-specific safety signals and pregnancy outcomes.
Figure 4: Abortions in England and Wales for fetal anomalies between March and May
Formal responses
The Paul-Ehrlich-Institut produced a statement in August 2023. They claimed the number of reports exactly correlated with doses given for every batch in their analysis of a prospective survey of the vaccinated called SafeVac 2.0. What they did not make clear was whether the early batches were included in this analysis or not. Their evidence does indicate that differences in comorbidities and age between batches has minimal impact on reporting rates. The Danish authors have critiqued this report and put forward powerful evidence that instead of the PEI using a denominator of all those given a dose they used a denominator of all those harmed by a dose. Inevitably the result was a linear relationship of reports to doses.
The Danish Medicines Agency carried out an analysis. They too found outliers among early batches but attributed this to heightened reporting by healthcare workers and the elderly. The booster doses given in care homes in Autumn 2021 did not cause further outliers in the data.
Figure 5: Reports to Danish authorities by batch and time
Unanswered Questions
The Pfizer vaccine batch discrepancies and associated defenses leave significant unanswered questions:
Testing Gaps: Why were some of the safest batches not subjected to rigorous quality control?
Global Overlaps: Why do problematic batches flagged in Denmark overlap with those flagged in the Czech Republic and confirmed by Swedish data?
Regulatory Deflection: Why did regulatory authorities rely on a student’s critique and fail to directly address the safety concerns raised by independent studies?
Fetal Anomalies: What is the potential connection, if any, between early batches and the spike in abortions for fetal anomalies observed in March–May 2021?
Conclusion
The Pfizer vaccine batch scandal highlights troubling inconsistencies in manufacturing, quality control, and regulatory oversight. The reliance on figures like Borja Somovilla del Saz, coupled with insufficient explanations from BioNTech, Pfizer, and the PEI, underscores systemic issues in how pharmaceutical safety concerns are managed and communicated. As the debate continues, the need for accountability, transparency, and rigorous inquiry into vaccine safety signals remains critical.








"As the debate continues, the need for accountability, transparency, and rigorous inquiry into vaccine safety signals remains critical". My question is has there ever been accountability, transparency and rigorous inquiry in the history of any vaccine?
Speaking to regulatory oversight; In the U.S. the Department of Justice administer the laws involving charges against U.S. sited industries. Pfizer has since 2008, paid over $3.6 billion in total fines with $1.3 billion being fines for criminal charges. Most fines were for bribes and kickbacks to doctors. "Regulatory capture" is clearly in evidence in that all penalties issued were a function of what is essentially no contest pleas where no guilt is admitted and the corporate guilty NEVER do jail time. Nor do the so called "doctors" who took the bribes. In fact we never know the names of the physicians involved in the scheme. My thought is that these "doctors" were some of the same ones who pushed the questionable COV2 injections WITHOUT informing their patients of the risk/benefit profile. I might further assert that MOST Docs didn't even look up the REAL risks listed on the Pfizer website PRIOR to the easily foreseen rollout in 2022, never mind informing their trusting patients. The U.S. DOJ is running a protection racket, but I also believe every physician responsible for signing off on an injection either IN IGNORANCE of the risks or especially when they ARE AWARE of the risks and without obtaining informed consent, should be seriously examined for malpractice by some other layer of regulator like a medical board.
On a slightly different topic, one might ask if any of the fines paid by any or all of the Pharma guilty parties ( Pfizer, Moderna, AZ,Bayer et al) over the decades go directly to the VACCINE INJURED? Of any modern era?